Latvian Biomedical Research and Study Center continues involvement of participants in the Latvian Population Genome Reference Project, which takes place within the framework of the European ‘1+ Million Genomes’ initiative
The main goal of the project is to create a genome reference of the Latvian population that will be used for disease prognostication, screening planning, treatment optimization, and scientific development. The Latvian genome reference created as part of the project, together with the population genome data of other European countries, will be included in the unified European genome database within the framework of the ‘1+ Million Genomes’ (1+MG) initiative. The goal of the 1+MG initiative is to provide secure access to genetic and sample-related clinical data across Europe to improve research, develop personalized healthcare, and support more effective decision-making in healthcare policy (more information on the 1+MG initiative here: https://digital-strategy.ec.europa.eu/en/policies/1-million-genomes).
Participant selection
Every adult citizen of Latvia is invited to participate in the project. However, for the genome reference of the population of Latvia to reflect the population of Latvia as accurately as possible, the involvement of participants will be carried out by ensuring an even distribution of gender, nationality and place of birth in accordance with the demographics. It should be emphasized that for the genome reference to be valid, two or several first-degree blood relatives may not participate in the project. A total of 3,500 participant enrollment is planned in the project.
Procedure for participation in the study
After filling out your application form, the project organizer team within 30 days will evaluate the information you provided and the compliance with the research selection criteria, and provide you with an answer by e-mail. If your participation in the study will be approved, you will receive information on the sample submission procedure in the confirmation e-mail. The e-mail will include:
- A unique link to a questionnaire with questions about your medical history and lifestyle;
- A print document file, which will contain a referral to E. Gulbja Laboratory and your unique informed consent documents;
- An information file containing information sheets approved by the Central Medical Ethics Committee, which must be read before signing the informed consent.
The print document file will contain a referral to E. Gulbja Laboratory, informed consent documents for participation in the Latvian Population Genome Reference Project (2 copies) and the Genome database of Latvian population (2 copies). Please read, print, fill out these documents carefully, sign them and take them along with your passport or ID to the sample delivery location – the E. Gulbja Laboratory branch that is most convenient for you. The amount of blood required in the project is small (10 ml), so participation in the project can be combined with other analyses. To ensure successful DNA extraction, it is recommended to donate blood no earlier than 3 hours after the last meal and refrain from eating fatty foods.

Why is it important to study the genome of the population of Latvia and create a genome reference for the Latvian population?
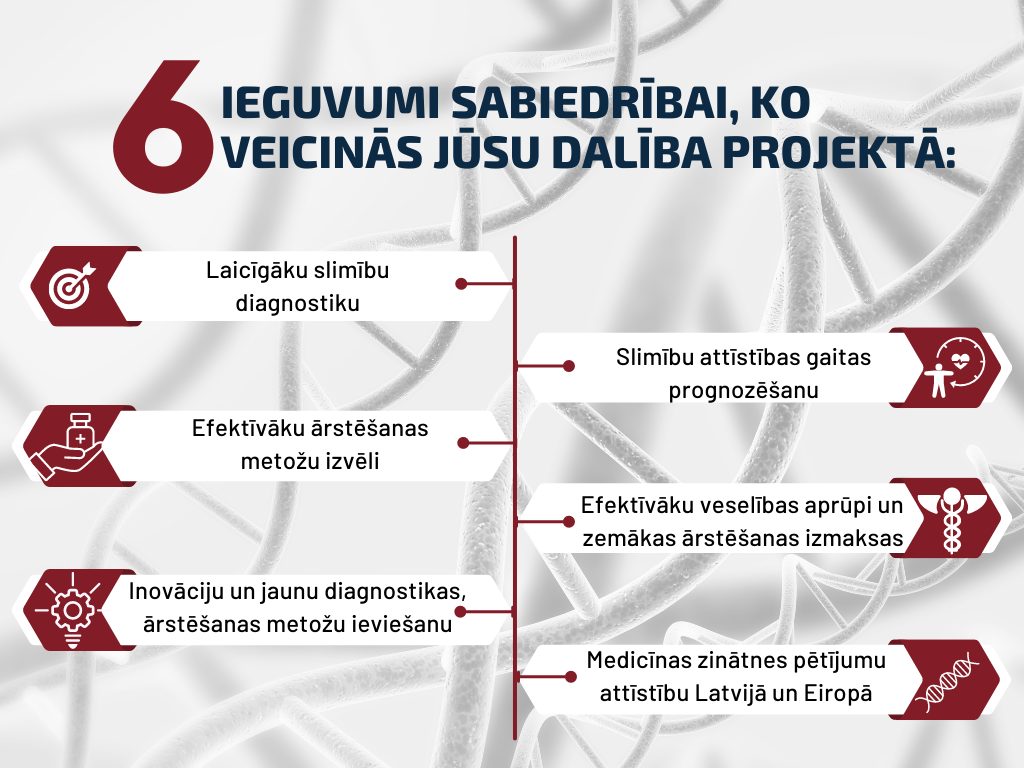
Extensive research worldwide and the ever-lowering costs of DNA sequencing have contributed to a paradigm shift in healthcare towards precision medicine. A genome reference is a standardized representation of the human genome that is used for comparison with the patient’s DNA every time doctors or scientists interpret DNA sequencing or determined nucleotide sequence data. It has been proven that genetic factors affect the development of many diseases, therefore, by taking them into account, the diagnosis can be made earlier and more accurately, thus increasing the chances of a successful therapy outcome. Similarly, using the genetic information can lead to more precise selection of drugs and therapies, since in most cases the effectiveness of drugs and the risks of side effects are also determined by genetic factors. However, even though the genome (complete set of genes) is the same for all people, there are significant differences in the nucleotide sequence of DNA between different ethnic groups and populations that can affect a person’s physical appearance, health, and incidence and manifestation of diseases. During genetic testing, the DNA nucleotide sequence obtained from the patient is compared to a genomic reference, which allows identification of differences in the nucleotide sequence, providing information on their clinical significance (beneficial, neutral, likely pathogenic, or pathogenic single nucleotide variation). Currently doctors and scientists in Latvia interpret results of genetic analyses using genome reference databases that do not include the genome data of Latvian residents. The lack of genomic data of the Latvian population in reference databases can create a situation where changes in the DNA nucleotide sequence identified in an individual are interpreted incorrectly, thus increasing the risk of drawing false conclusions about the genetic causes of the disease, while at the same time hindering the improvement of health care in general. Looking at it more broadly – in the context of Europe and the world – greater diversity in genomics research has several advantages, starting with new discoveries about differences in health status, better understanding of human biology, which will also improve healthcare options, and finally, more precise and extensive use of genetic testing.
Processing of samples and documentation
The confidentiality of the project participants is ensured in accordance with the General Data Protection Regulation and the Personal Data Processing Law. Blood samples from all study participants, as well as information from the health and heredity questionnaire, will be coded, replacing personal data with a unique code, which means that all information will be pseudo-anonymized and access to personal data will be strictly limited. In this way, scientists and doctors who study the information and samples from the database, will not have access to the personal data of the project participant.
Other information
The research protocol has been reviewed and approved by the Central Medical Ethics Committee (approval no. 01-29.1.2/1669) and the Genome Research Council (opinion no. A-5/22-05-24).
Contact information
For cooperation opportunities, please write to Janis Klovins (leading researcher) at klovins@biomed.lu.lv.
For questions related to participation in the project, please write to genoma-projekts@biomed.lu.lv.

The project is financially supported by the European Recovery Fund (project No. 4.1.1.r.0/3/22/I/VM/001).